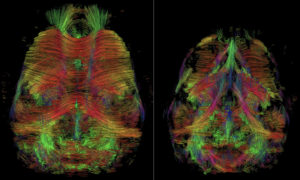
Diffusion tensor imaging tractography in wildtype (left) and Wdr47 full knockout (right) mice. By Chrystelle Po (ICube, University of Strasbourg, CNRS, FMTS, Strasbourg, France).
Scientific orientations
In vivo modelling of human neurodevelopmental disorders is important in basic and clinical research since the brain cannot be easily accessed in humans. Humans and mice share neurodevelopmental principles, and whilst the cortical folding of the human brain is absent in the naturally lissencephalic mouse brain, brain anatomy is conserved across these two species. To study the phenotypic effects of genes and variants at the whole organism level, we and others turned to the long-standing mouse model, where pleiotropy can be studied and where both environment and genetics are controlled. With its extensive toolkit and ability to recapitulate human neurodevelopmental disorders, the laboratory mouse offers a number of powerful tools to make association between phenotypes and genotypes and for studying and validating the effect of human genetic variants using CRISPR–Cas9 editing technology. Our increasingly sophisticated ability to phenotype the mouse brain, combined with ultra-standardized 2D neuroanatomical pipelines, argues that variant discovery in mice will remain crucial to progress in the post-genomic era.
Specific aims
We focus on the poorly characterized WD40-repeat (WDR) genes, one of the largest eukaryotic gene families, in corpus callosum biology and neuronal connectivity.
Our lab is interested in three main questions:
-
What WDR genes shape our brain ?
-
What WDR variants lead to human neurodevelopmental disorders ?
-
How WDR genes and variants lead to human neurodevelopmental disorders ?
Our hypothesis is that the miswiring between neurons may contribute to the pathogenesis of neurological symptoms in “WDRopathies”.
Perspectives:
-
Identify novel genes that regulate brain anatomical changes differentially in male and female using large-scale mouse knockout screens.
-
Determine the function of novel genes associated with sexually-dimorphic neuroanatomical differences at the cellular, physiological and behavioural levels.
-
Decouple the effects of gonadal hormones from sex chromosomes using the Four Core Genotype strategy, a powerful and unique tool to the mouse.
-
Explore undetermined female compensatory programs by implementing a new technique within the nuclei of individual brain cells where sex chromosomes and sex hormone receptors reside.
